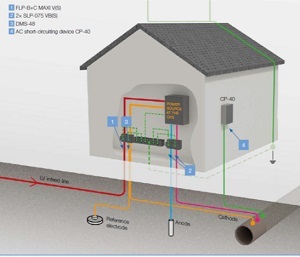
The commodities that are typically moved are fossil fuels, namely oil and oil products, gas, etc. The most efficient way to move these commodities is through 'product pipelines', i.e. oil and gas pipelines, which are large pipelines buried in the ground.
Pipelines are usually made of steel and, even when protected by a layer of insulation, can be damaged locally, leading to corrosion of the pipeline in wet soil and, as a result, the risk of fatal accidents.
In order to prevent corrosion of steel pipes, in various environments defined by electrochemical stability.
The various electrochemical properties of iron are utilized. 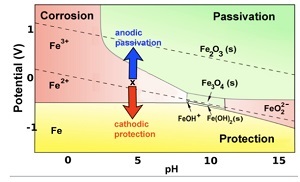
Iron diagram (so-called Pourbaix diagram).
The diagram shows that if the potential of the protected object made of iron can be permanently maintained at minus 0.7 V above the ambient level, the iron will function reliably and will not corrode even in a humid environment.
This method of corrosion protection is called "cathodic protection" because the object being protected is: 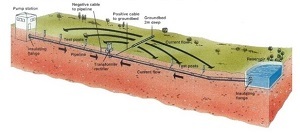 In essence, it becomes the cathode of an artificially created galvanic cell. In practice, this protection is provided by an automatic DC power system, one pole (cathode) of which is connected to the protected object (pipe) and the other pole (anode) is introduced in the form of an anode rod into the soil surrounding the pipe.
In essence, it becomes the cathode of an artificially created galvanic cell. In practice, this protection is provided by an automatic DC power system, one pole (cathode) of which is connected to the protected object (pipe) and the other pole (anode) is introduced in the form of an anode rod into the soil surrounding the pipe.
The controller then protects the pipe from corrosion by keeping the voltage potential of the pipe below -0.7 V. However, this means that a certain amount of electricity must be supplied to this system permanently, i.e. a certain operating cost.
Then the situation worsens dramatically in areas where high-voltage lines are located near protective pipelines (or other steel components). 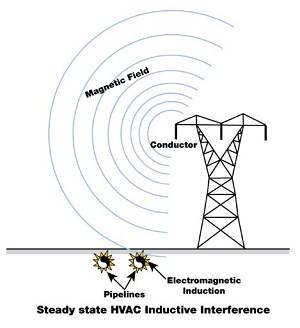
The magnetic field generated during HV line operation induces a secondary alternating voltage in the nearby pipelines, which is superimposed on the DC voltage of the cathodic protection already mentioned.
In the better case, automatic regulation of the pipeline voltage potential means that much more energy is needed to compensate for the potential changes in the pipeline due to the induced AC voltage, i.e. much higher operating costs, and in the worse case, rapid corrosion, perforation and collapse of the pipeline occurs when the superimposed AC current reaches a level that cannot be compensated for.
Two signs of this undesirable phenomenon are enough to justify taking action to remedy the situation.
The solution consists in using special electrical filters that selectively remove the induced AC component from the affected pipe and return the cathodic protection to its normal maximum efficiency state (in terms of electrical potential and cost). 
Installing a filter in the cathodic protection system ensures safe operation of the cathodic protection (eliminating the risk of corrosion) and at the same time saves significant financial costs during the operation of the system. In addition, the potential breaker has an additional surge protection function against direct and indirect lightning, protecting not only the filter itself, but also the entire electrical part of the cathodic protection from damage due to voltage surges.
The corrosion protection device can be easily connected to an existing cathodic protection system and can eliminate AC induction currents of up to 40A peak! The installation of this pipeline means safe operation of the pipeline, especially in areas where the pipeline crosses or coincides with distribution lines, AC electric railway lines or other potential sources of AC current induced by the pipeline.
In addition to the filter, other parts of the cathodic protection system must also be protected from overvoltage, as shown in the following figure.